Smile Quality

TIQCon (Total Integrated Quality Control)
Inter Laboratory Quality Control benchmark program
Laboratory testing of patient samples can be a complex procedure, depending on the clinical analysis, a microbiological study or blood bank testing in all areas of the clinical laboratory. Quality Control (QC) is one of the most important effects on laboratory tests – ensuring both precision and accuracy of patient sample results. The integrity of quality control samples is important for both overall quality management and fulfillment of proficiency testing requirements. Addressing QC issues is critical to identifying potential errors in patient outcomes.
If quality control works effectively, it can find errors in a lab’s analytical processes and help correct them before potentially publishing incorrect patient results. Clinical laboratories use documentation management and the integration of a continuous improvement process to streamline the overall quality control process.
Another way to analyze quality control is peer testing and monthly review of QC trends. Clinical laboratories are often involved in clinical laboratory tests (PT) that validate their QC evaluations. Not the result of a single laboratory, but the comparison with a peer group provides security in the validation of QC results. Periodic review of QC results is a common tool for maintaining quality control of patient samples.
For this procedure TIQCon is the ideal tool!
TIQCon allows clinical laboratories to evaluate the performance of their assays on the basis of a statistical analysis of QC data generated by the control manufacturer for Chemical, molecular and/or clinical immunochemical analysis devices.
TIQCon collects QC data from different customer controls (from different countries) using the same batch of control material, the same type of analyzer and the same analyte.
TIQCon is independent from any control material manufacturer
TIQCon evaluates your quality data according to different standards such as SFBC, Wetgard etc.
TIQCon captures QC data from almost any device via a hardware or software solution, or receives its QC data via standardized transfer formats (XML).
TIQCon Quality Desktop, a very user-friendly interface.
TIQCon works as a desktop application and offers a variety of evaluation options with the simplest application.
Startpage view
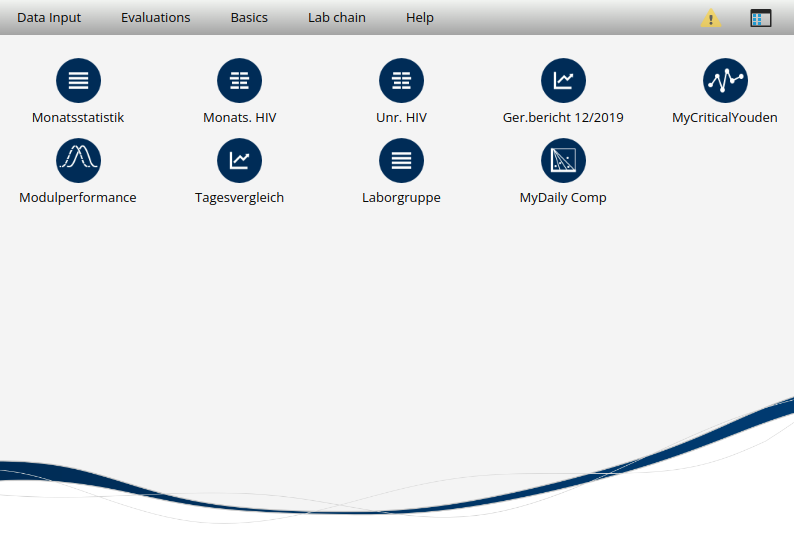
very simple overwiew of user specific desktop shortcuts
Current control cycle
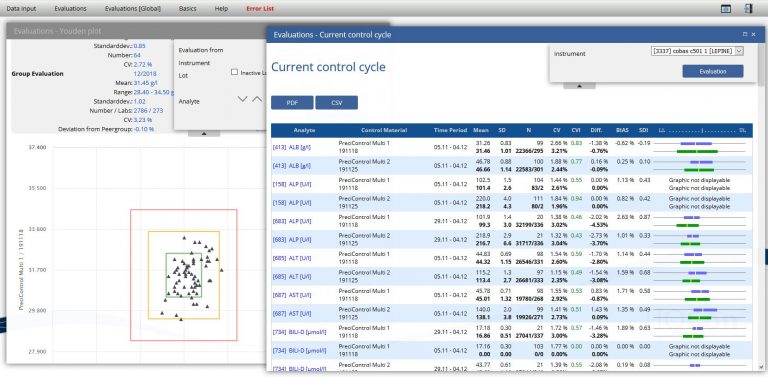
Fast analyzer based overview about current statistics
Multiple windows can be opened inside the application this helps to compare evauations.
Levey Jennings / multi level chart
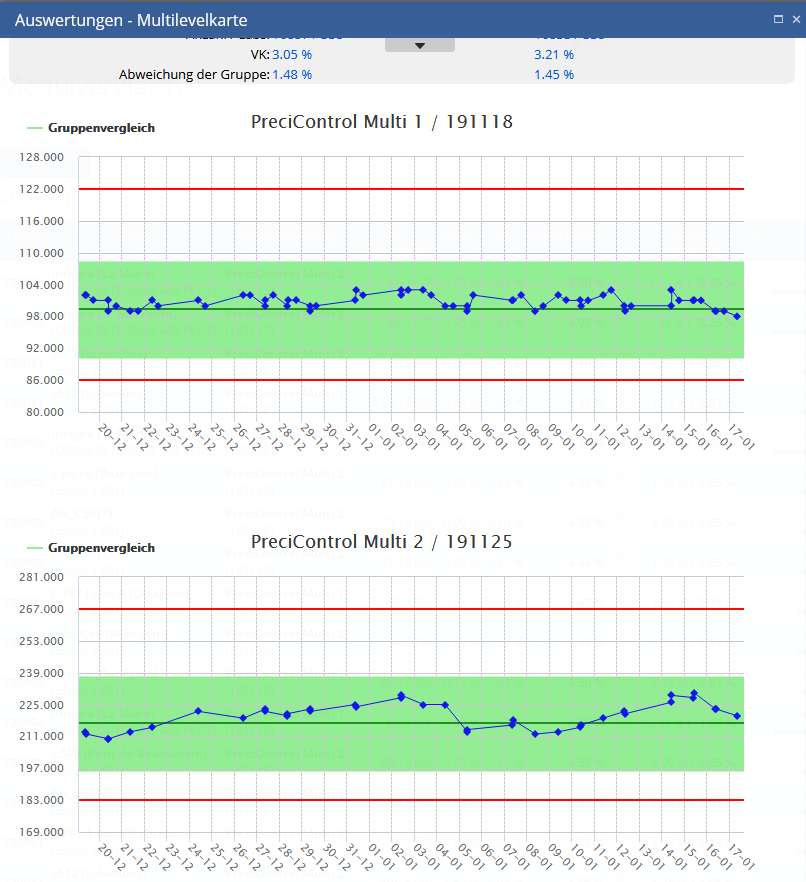
Analytical LJ charts will help to compare analyzer against peergroup benchmark
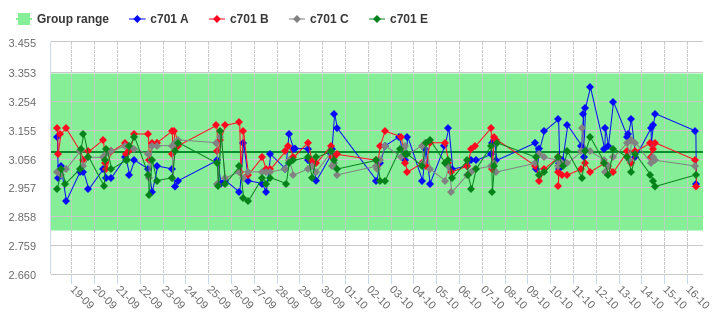
Peergroup benchmark
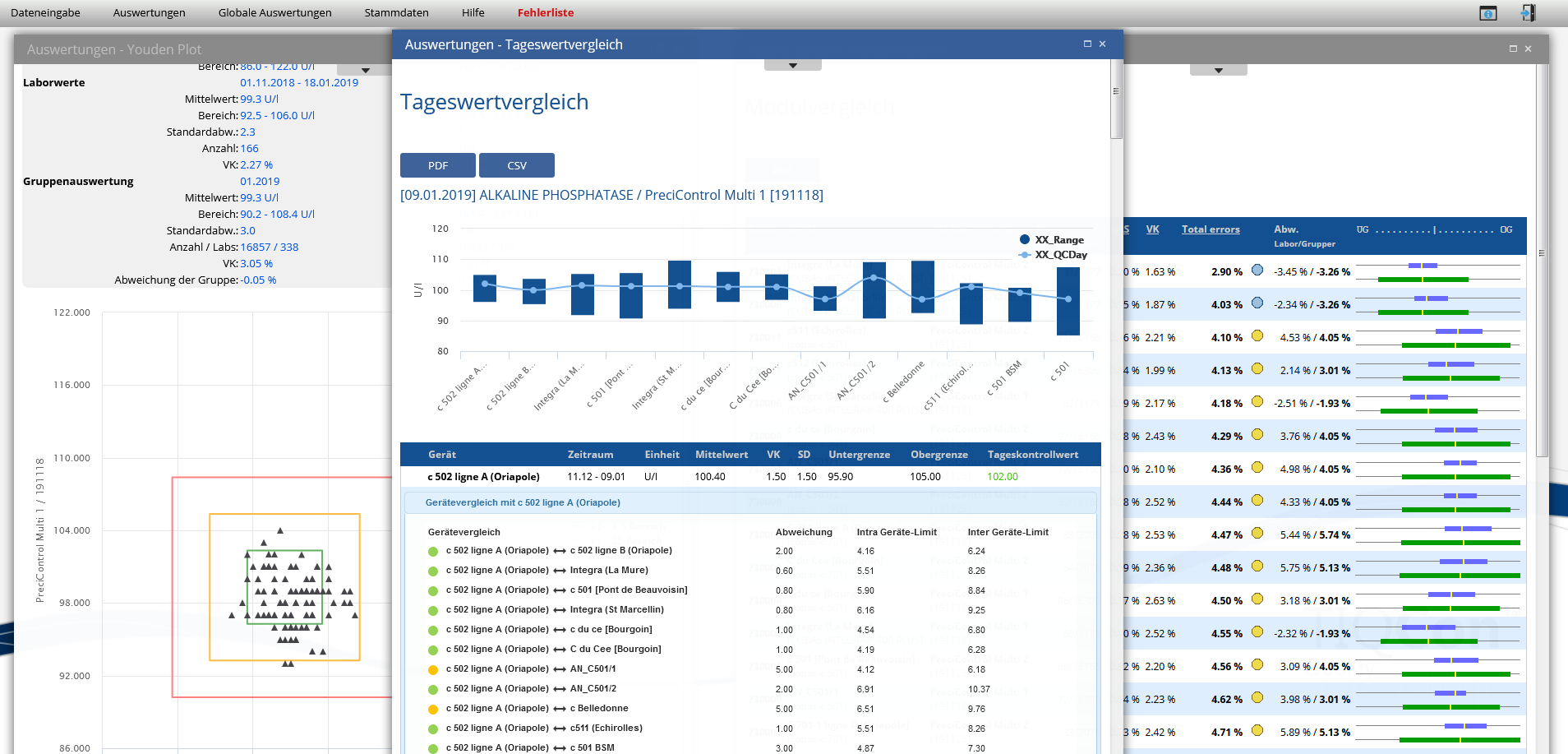
Monthly based overview lab vs peergroup of all analytes
Youden plot
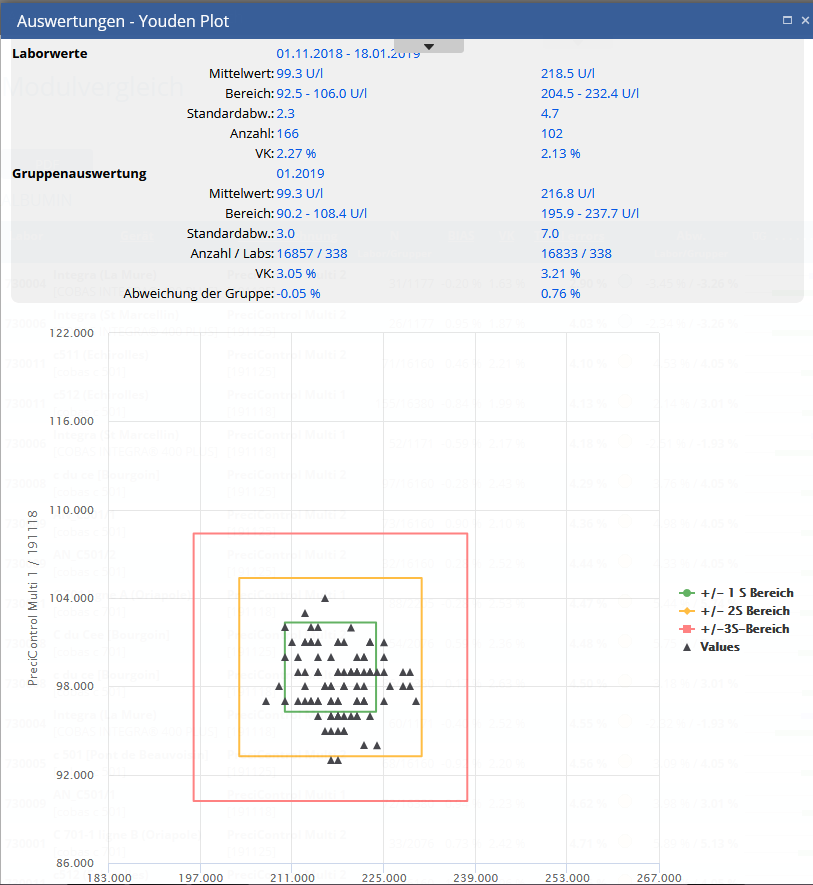
Graphical representation of 2 level QC in +/- 3 SD
Module Performance
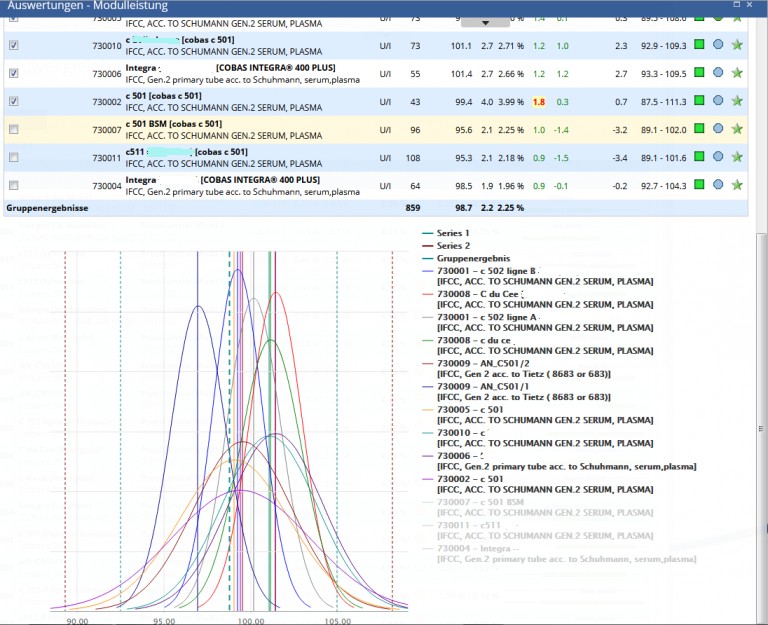
Instrument statistics
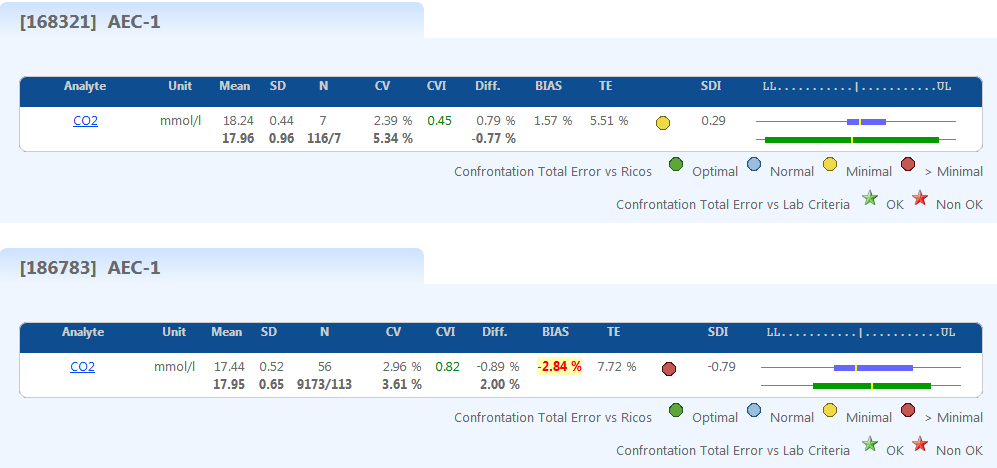
Uncertainty
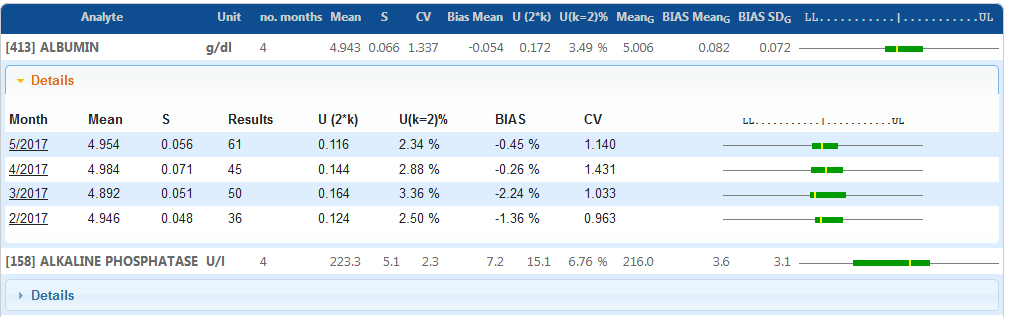
Monthly based uncertainty calculation compared to previous months
OPS chart SIX sigma
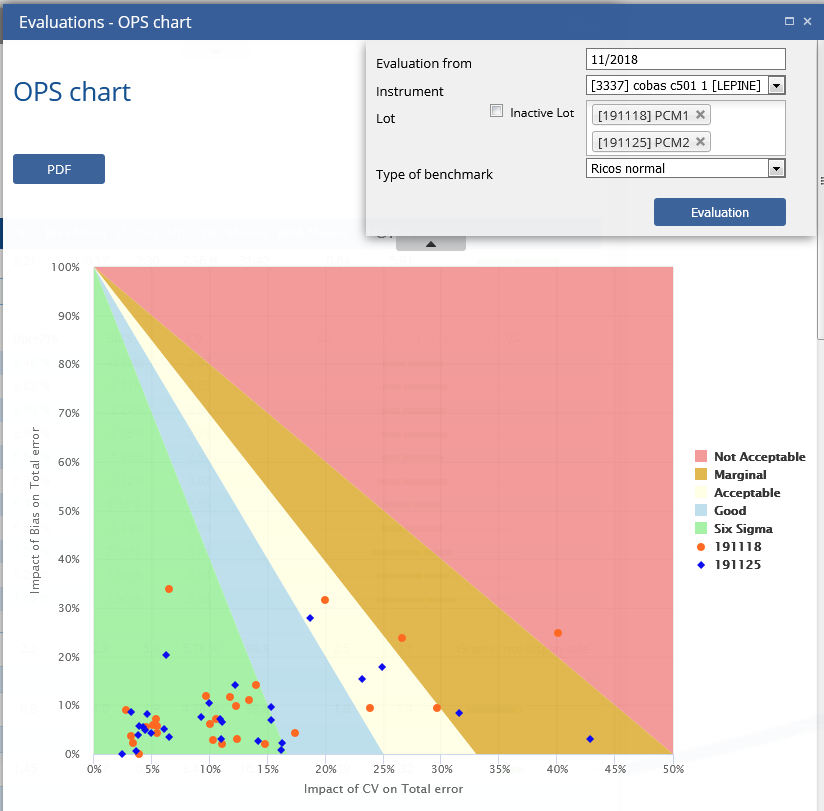
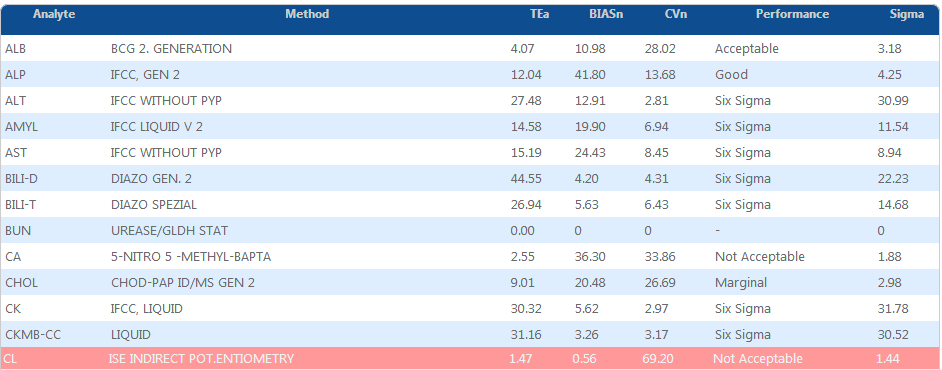
TIQCon is the most efficient and user-friendly quality control system for clinical laboratories.
- Compares the internal QC with the peer group’s cue point.
- Cloud-based Solution, connectivity and data collection solutions…
- Almost any device or analysis protocol can be connected.
- Used for clinical chemistry, immunodiagnostics or molecular diagnostics. Batches of control materials with their nominal values can be imported and used all over the world.
- Can send information and warnings directly via email
- Lab chain management
- Characteristics such as BIAS, CVI, TE and measurement uncertainties U (K = 2) and U% are available on the fly
- Laboratory-specific limits for u.a. TE, BIAS, CV and biological impact
TIQCon is used worldwide as an instrument for accreditation of laboratories and keeps you informed !